The Council for Medical Schemes (CMS) has slammed allegations by the Board of Healthcare Funders (BHF) that it is deliberately sabotaging medical schemes to boost the perceived value of National Health Insurance (NHI).
The 31-page rebuff follows “a deliberately leaked” lawyer’s letter sent to the CMS by the BHF in February. In the letter, the BHF takes the Registrar of Medical Schemes, Dr Sipho Kabane (pictured), and the CMS to task for allegedly displaying hostility towards medical schemes and of acting in ways that undermine the industry.
The BHF, a non-profit company, says its membership consists of about 40 schemes and medical scheme administrators that serve some 4.5 million beneficiaries.
The two entities have been at loggerheads for years, specifically over the delay in implementing a regulatory framework for low-cost benefit options (LCBOs). This was but one of the areas of conflict laid bare in the lawyer’s letter.
Read: Board of healthcare funders takes Council for Medical Schemes to task in lawyer’s letter
The CMS comes out swinging in its response, describing the BHF leadership’s “combative conduct” in addressing its concerns with the Minister of Health, the chairperson of the CMS, and through the media “as extreme, bizarre, and not in accordance with common sense, as well as the prescripts of the Medical Schemes Act (MSA) and regulations”.
Instead of creating a “media circus”, the CMS asks why the BHF did not follow the remedies (found in chapter 10 of the MSA) for alleged unwarranted conduct that is ultra vires by the Office of the Registrar.
The regulator said while the BHF’s “reason for its sole existence” is to represent medical schemes and administrators, the CMS represents the interest of the country’s 9.04 million medical scheme beneficiaries. It follows, the CMS says, that the entities’ mandates and vested interests will not always coincide.
“The CMS is therefore not surprised that the BHF would place their narrow commercial and vested interests of its members (medical schemes, administrators, and others) above those of the 9.04 million medical scheme beneficiaries of medical schemes,” the CMS states.
NHI informing CMS policy prematurely?
One of the BHF’s key concerns is whether the NHI Bill has been influencing CMS policy decisions prematurely.
The lawyer’s letter says the BHF has reason to believe that the NHI Bill has informed the CMS’s approach to regulation for the past five years, even though the Bill was approved by the National Council of Provinces only in December 2023 and has not been assented to by the President.
The CMS pans the BHF’s narrative – which suggests that the National Department of Health (NDOH) influences the CMS – as a misunderstanding of the regulator’s governance structure.
The CMS says it operates as a statutory body under the MSA and is classified as a schedule 3A public entity according to the Public Finance Management Act (PFMA).
It goes on to explain that as such, the CMS has its own governing body, referred to as the council, which serves as its accounting authority.
The report states that, according to section 7 of the Act, the council advises the Minister of Health on national health policy matters, and the minister may assign tasks to the council as deemed necessary.
Under the PFMA, the minister acts as the executive authority of the CMS, presenting its budgets and plans to Parliament.
“The relationship that exists between the CMS and the NDOH is not based on personal whims but is established by law,” the CMS states.
The CMS asserts that allegations it acts under the minister’s guidance are unfounded, emphasising the clear lines of reporting in the MSA and the PFMA.
“It appears that the BHF is mainly concerned and seeks to challenge the health-policy direction that the NDOH under the leadership of the Minister of Health is taking. It should be noted that, despite its many utterances to the contrary, the BHF is not in support of the NHI,” the report notes.
‘Unnecessary and expensive litigation’
Eight pages of the CMS report address the “bullying of medical schemes” and “curatorship”.
The BHF accused the CMS of using expensive lawsuits and curatorships to intimidate medical schemes, hindering their operations. It criticised the registrar’s frequent use of curatorships and alleged a pattern of targeting smaller schemes, potentially forcing them to close or merge. It also raised concerns about wasteful spending on legal battles and demanded transparency on litigation outcomes.
The CMS vehemently denies the allegations of bullying schemes into extinction or forced mergers. Regarding curatorships, CMS said only a court has the authority to appoint a curator, not the CMS or the registrar.
The CMS states that its regulatory interventions are driven by its mandate in section 7 of the Act, namely “to always protect the interest of beneficiaries”.
The registrar is expected to make a case to the council to obtain concurrence.
“The BHF appears to bestow the powers of placing medical schemes under curatorship to either the Office of the Registrar or the council, and this is a clear misunderstanding of the application of the relevant legislation,” the report reads.
Statistics show that between 2012 and 2022, 11 schemes were placed under curatorship, mostly because of governance issues.
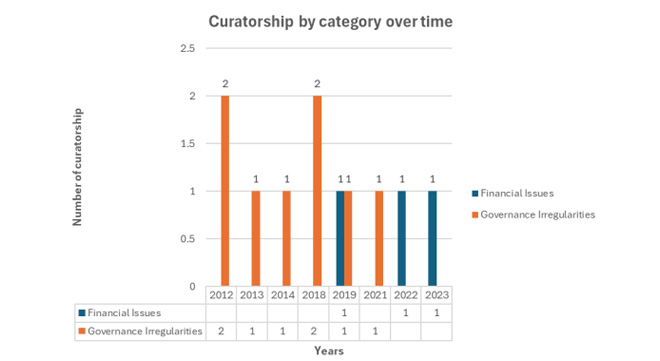
The CMS argues that in proportion to the total number of medical schemes during the same period, this represented about 14% of all schemes.
“In terms of several lives covered, the affected schemes accounted for 6% of the medical scheme industry. These statistics highlight the insignificant impact on affected schemes, countering the perception that regulators act hastily in placing schemes under curatorship.”
Curators are appointed by the court, not the CMS, and they can only be removed by the court. In urgent cases, an ex parte process allows for immediate intervention, but the CMS emphasises this is a legal remedy, not a decision made by it.
Between 2012 and 2023, the CMS placed seven medical schemes under curatorship through ex parte procedures, with one application rejected by the court. Additionally, four schemes were placed under curatorship through a notice process during the same period.
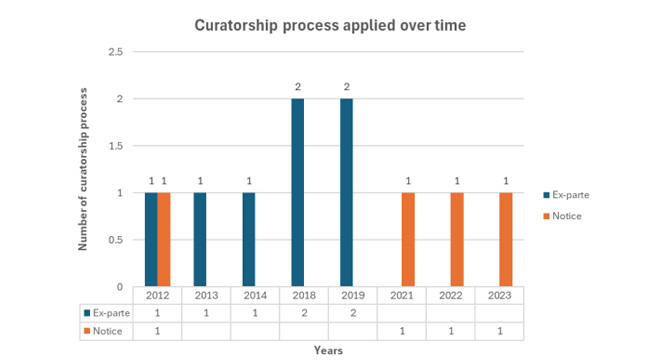
The CMS argues that this disproves the notion that its applications for curatorship are typically ex parte. It asserts that the accusations regarding curatorship are unfounded, noting that ex parte applications are common legal remedies.
“If there is a shift not to apply ex parte applications, then changes to the law have to be made, but, unfortunately, CMS is not a policy- nor a law-maker.”
The CMS responds to the BHF’s accusation of wasteful spending by highlighting its “exceptional organisational performance”, supported by a clean audit from the Auditor-General and an overall performance score of 89.9%.
In the report, the Office of the Registrar requests evidence that small schemes are being forced to merge.
“We also wish to place on record that there have not been any appeals that have been lodged with the different appeal structures at the CMS to address any matters related to the misconduct of the Office of the Registrar.”
Delays in implementing low-cost options
For seven years, LCBOs have caused conflict between the CMS and medical schemes. LCBOs include cheap, pared-down packages that do not provide all the benefits required by the MSA.
Schemes will require exemptions from the Act’s requirements to offer these slimmed-down benefit options. Since 2016, the CMS has been working on developing a legal framework that will enable medical schemes to offer LCBOs.
In 2022, BHF took the regulator to court, accusing it of delaying the implementation of LCBOs.
Read: BHF takes legal action against the CMS to compel it to review the LCBO guidelines
Since the BHF filed its main application in 2022, it has brought further applications concerning the record relating to the CMS’s and the Minister of Health’s decisions on LCBOs.
Despite a judgment handed down in July last year to the CMS and the Minister of Health to produce all the documents listed by the BHF, none has yet been provided.
The litigation between the BHF and the CMS continues.
Read: CMS seeks reconsideration after court dismisses appeal on LCBO documents
In the letter, BHF (again) accuses the CMS of delaying the LCBO guidelines, harming scheme viability, and member affordability. It (again) requests a copy of the registrar’s LCBO report, which was submitted to the minister in November 2023.
In response, the CMS says the minister has authority over the report, and stakeholders must await his decision.
“CMS should not and will not share the contents of this LCBO report until CMS has been specifically furnished with instructions and/or permissions to do so by the Minister of Health.”
According to the CMS, the repeated requests for the LCBO report undermine the minister’s decision.
“It should also be noted that their (BHF) position in respect of the LCBO is to argue for its introduction irrespective of the impact that this may have on the entire health system and the 62 million of the South African population,” the report reads.
Threat to approach the Public Protector
The BHF gave the CMS and the minister until 8 March to respond to the issues raised in the letter. The BHF said it would complain to the Public Protector if its concerns were not addressed “promptly and adequately”.
The Office of the Registrar responded to the letter and allegations on 26 April, expressing their “right of reply to the various falsehoods perpetuated in the leaked letter by BHF”.
“We take note of the threat by the BHF to lodge a complaint with the Public Protector should they not be satisfied with the way the council will address these complaints and allegations. We, however, believe that this veiled threat to the council and the minister is pre-emptive, unjustified, and unfair. This approach reveals the true intention of this letter by the BHF, which is to publicly tarnish the reputation of the CMS for reasons best known to them,” the report reads.
To download the full report, click here.



